Client Papers | GMC team develops 18F-labeled peptide probes, Madic PET/CT facilitates accurate imaging of cancer fibroblasts
Release Date:2025-06-30 Number of views:277
Xinlu Wang's team at the Department of Nuclear Medicine, The First Affiliated Hospital of Guangzhou Medical University, published a study in EJNMMI Research, reporting for the first time the preclinical evaluation and first human imaging of the novel 18F-tagged fibroblast activation protein (FAP)-targeting peptide [18F] AlF-FAP-NUR, and the MadicLab PSA071 PET/CT system in an animal model The MadicLab PSA071 PET/CT system demonstrates high-resolution tumor visualization capabilities in an animal model, providing a new tool for precision cancer diagnosis and treatment.
Research Background and Technical Challenges
FAP is specifically highly expressed in tumor-associated fibroblasts (CAFs) and is an ideal target for tumor imaging. Although 68Ga-labeled FAP peptides (e.g., FAP-2286) have been used for PET imaging, the lack of supply of 68Ge/68Ga generators and the short half-life of the nuclide (68 min) have made it difficult to meet the clinical demand for high throughput.18F-labeled probes (half-life of 109.8 min), which can be mass-produced by the medical cyclotron and have a higher spatial resolution, have become an ideal alternative. The 18F labeled probe (half-life 109.8 min) can be mass-produced by medical cyclotron and has higher spatial resolution, making it an ideal alternative.
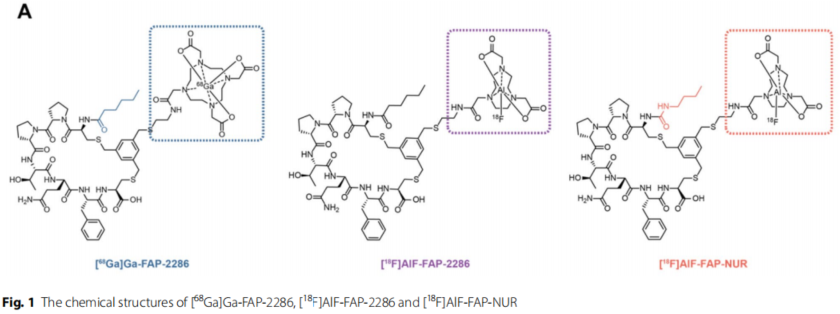
Figure 1: Chemical structures of [68Ga]Ga-FAP-2286, [18F]AlF-FAP-2286 and [18F]AlF-FAP-NUR
The central role of MadicLab PET/CT
1. Preclinical Animal Imaging: High Resolution Tumor Specific Uptake Assessment
Device Parameters
Using MadicLab PSA071 PET/CT with spatial resolution up to 200 μm supports dynamic PET scanning in mouse models.
Key Findings
In 293T-FAP loaded mice, tumor uptake of [18F]AlF-FAP-NUR (26.99% ID/g) was 2.3 times higher than that of 68Ga-FAP-2286 (11.55% ID/g), with tumor-muscle ratios up to 61.31 vs. 22.66, demonstrating a higher tumor-to-background contrast ratio6-77.
Dynamic imaging showed rapid clearance of the probe in the kidney (~50% clearance at 45 min) and low uptake in non-target organs such as muscle (0.35±0.13% ID/g at 60 min), confirming its favorable pharmacokinetic properties.
2. Molecular mechanism validation: cellular level uptake and metabolism analysis
CELLULAR ASSAYS: Quantitative analysis by MadicLab PET/CT showed that [18F]AlF-FAP-NUR had a significantly higher uptake rate in HT1080-FAP cells (65.77% ID/million cells) than 68Ga-FAP-2286 (42.47%) and a much lower rate of exocytosis (120-minute residue >52%).
Metabolic Stability: In vitro stability assays demonstrated that the probe had >90% radiochemical purity in serum at 37°C for 4 hours, supporting clinical translation.
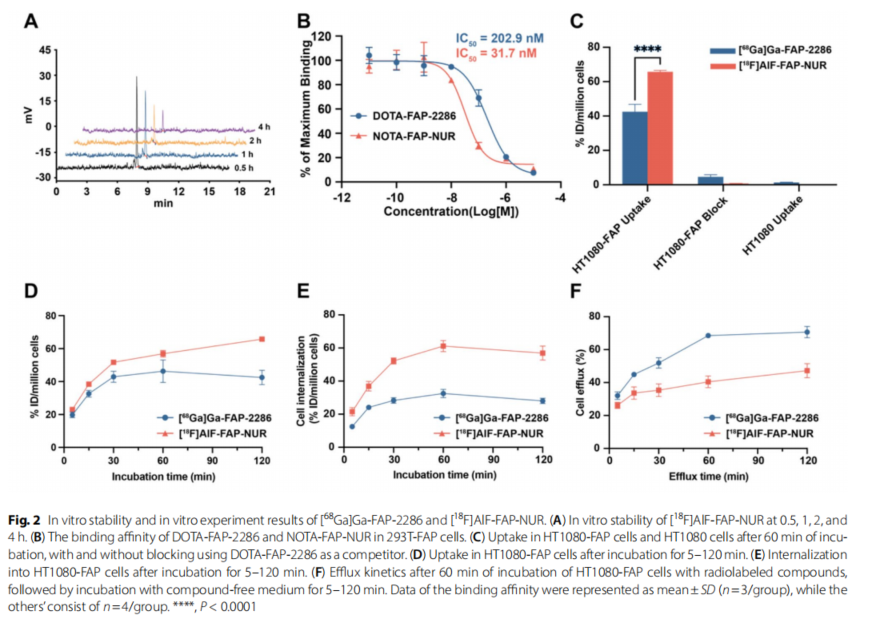
Figure 2: In vitro stability and in vitro experimental results of [68Ga]Ga-FAP-2286 and [18F]AlF-FAP-NUR. (A) In vitro stability of [18F]AlF-FAP-NUR at 0.5, 1, 2, and 4 hours. (B) Binding affinity of DOTA-FAP-2286 and NOTA-FAP-NUR in 293T-FAP cells. (C) Uptake of HT1080-FAP cells and HT1080 cells in the presence or absence of DOTA-FAP-2286 as a competitor after 60 min of incubation. (D) Uptake of HT1080-FAP cells after 5-120 min of incubation. (E) Internalization of HT1080-FAP cells after 5-120 min of incubation. (F) Exocytosis kinetics of HT1080-FAP cells incubated with radiolabeled compounds for 60 min followed by 5-120 min of incubation with compound-free medium. Binding affinity data are expressed as mean ± standard deviation (n=3 per group) and other data are n=4 per group. ****P<0.0001
Figure 3: Figure 4 Micro-PET images and time-activity curves of [68Ga]Ga-FAP-2286 and [18F]AlF-FAP-NUR in male tumor-bearing mice. (A,C) Uptake of [68Ga]Ga-FAP-2286 and [18F]AlF-FAP-NUR was assessed at 60 min in 293T-FAP and 293T loaded tumor-bearing mice (n=4 per group). (B, D) Uptake of [68Ga]Ga-FAP-2286 and [18F]AlF-FAP-NUR at 60 min was assessed in 293T-FAP and 293T homozygous tumor-bearing mice (n=4 per group).Time-activity curves in A-D show the accumulation of radiotracer in the different organs from 5-120 min after injection. (E) Tumor background ratio (TBR) of the two tracers in different xenografts (including 293T-FAP and 293T hormonal mice) at 60 min. (F) TBR of both tracers in different xenografts (including A549-FAP and A549-loaded tumor-bearing mice) at 60 min. All data are expressed as mean % ID/g ± standard deviation (n=4).
3. Clinical Translation: First Human PET/CT Imaging
Clinical Equipment: Human imaging was performed using the UMI Panorama system, but pre-existing animal model data provided a key reference for clinical dose optimization.
Patient results: PET/CT of 2 cancer patients (breast cancer, lung cancer) showed primary tumor SUVmax of 21.20 and 14.19, muscle SUVmean of only 0.65-1.32, and TBR of 10.75-32.62, which verified the tumor-targeting ability of the probes in humans.
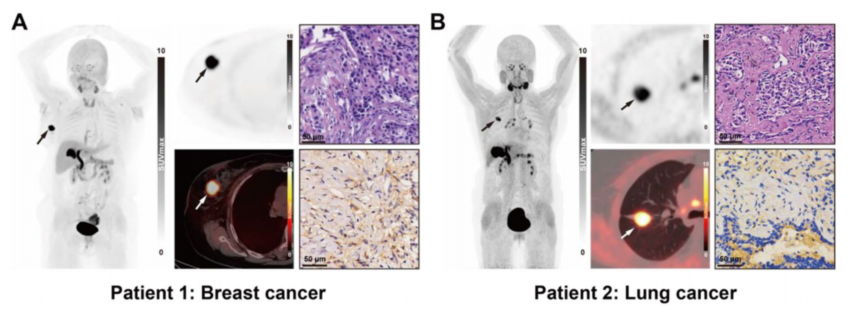
Figure 4 Clinical images of [18F]AlF-FAP-NUR PET/CT, HE staining results and FAP immunohistochemical staining of the patient's primary focus. (A) Patient with ductal carcinoma of the right breast. (B) Patient with invasive adenocarcinoma of the right lung. Scale bar, 50 μm.
Study Conclusions and Future Prospects
[18F]AlF-FAP-NUR combined with MadicLab high-resolution PET/CT demonstrated higher tumor-specific uptake and TBR values in animal models, and human imaging preliminarily confirmed its safety and efficacy. Although expanded clinical cohort validation is needed, this technology provides a new paradigm for early diagnosis and efficacy monitoring of FAP-positive tumors. In the future, it can be combined with the dynamic imaging capability of the MadicLab device to further optimize the pharmacokinetics of the probe and promote the development of precision nuclear medicine therapy.
Link to paper: DOI: 10.1186/s13550-024-01139-w
Equipment Support: MadicLab PSA071 PET/CT System (Shandong Madec Yinghua Technology Co., Ltd.)
Xinlu Wang's team at the Department of Nuclear Medicine, The First Affiliated Hospital of Guangzhou Medical University, published a study in EJNMMI Research, reporting for the first time the preclinical evaluation and first human imaging of the novel 18F-tagged fibroblast activation protein (FAP)-targeting peptide [18F] AlF-FAP-NUR, and the MadicLab PSA071 PET/CT system in an animal model The MadicLab PSA071 PET/CT system demonstrates high-resolution tumor visualization capabilities in an animal model, providing a new tool for precision cancer diagnosis and treatment.

Research Background and Technical Challenges
FAP is specifically highly expressed in tumor-associated fibroblasts (CAFs) and is an ideal target for tumor imaging. Although 68Ga-labeled FAP peptides (e.g., FAP-2286) have been used for PET imaging, the lack of supply of 68Ge/68Ga generators and the short half-life of the nuclide (68 min) have made it difficult to meet the clinical demand for high throughput.18F-labeled probes (half-life of 109.8 min), which can be mass-produced by the medical cyclotron and have a higher spatial resolution, have become an ideal alternative. The 18F labeled probe (half-life 109.8 min) can be mass-produced by medical cyclotron and has higher spatial resolution, making it an ideal alternative.

Figure 1: Chemical structures of [68Ga]Ga-FAP-2286, [18F]AlF-FAP-2286 and [18F]AlF-FAP-NUR
The central role of MadicLab PET/CT
1. Preclinical Animal Imaging: High Resolution Tumor Specific Uptake Assessment
Device Parameters
Using MadicLab PSA071 PET/CT with spatial resolution up to 200 μm supports dynamic PET scanning in mouse models.
Key Findings
In 293T-FAP loaded mice, tumor uptake of [18F]AlF-FAP-NUR (26.99% ID/g) was 2.3 times higher than that of 68Ga-FAP-2286 (11.55% ID/g), with tumor-muscle ratios up to 61.31 vs. 22.66, demonstrating a higher tumor-to-background contrast ratio6-77.
Dynamic imaging showed rapid clearance of the probe in the kidney (~50% clearance at 45 min) and low uptake in non-target organs such as muscle (0.35±0.13% ID/g at 60 min), confirming its favorable pharmacokinetic properties.
2. Molecular mechanism validation: cellular level uptake and metabolism analysis
CELLULAR ASSAYS: Quantitative analysis by MadicLab PET/CT showed that [18F]AlF-FAP-NUR had a significantly higher uptake rate in HT1080-FAP cells (65.77% ID/million cells) than 68Ga-FAP-2286 (42.47%) and a much lower rate of exocytosis (120-minute residue >52%).
Metabolic Stability: In vitro stability assays demonstrated that the probe had >90% radiochemical purity in serum at 37°C for 4 hours, supporting clinical translation.

Figure 2: In vitro stability and in vitro experimental results of [68Ga]Ga-FAP-2286 and [18F]AlF-FAP-NUR. (A) In vitro stability of [18F]AlF-FAP-NUR at 0.5, 1, 2, and 4 hours. (B) Binding affinity of DOTA-FAP-2286 and NOTA-FAP-NUR in 293T-FAP cells. (C) Uptake of HT1080-FAP cells and HT1080 cells in the presence or absence of DOTA-FAP-2286 as a competitor after 60 min of incubation. (D) Uptake of HT1080-FAP cells after 5-120 min of incubation. (E) Internalization of HT1080-FAP cells after 5-120 min of incubation. (F) Exocytosis kinetics of HT1080-FAP cells incubated with radiolabeled compounds for 60 min followed by 5-120 min of incubation with compound-free medium. Binding affinity data are expressed as mean ± standard deviation (n=3 per group) and other data are n=4 per group. ****P<0.0001
Figure 3: Figure 4 Micro-PET images and time-activity curves of [68Ga]Ga-FAP-2286 and [18F]AlF-FAP-NUR in male tumor-bearing mice. (A,C) Uptake of [68Ga]Ga-FAP-2286 and [18F]AlF-FAP-NUR was assessed at 60 min in 293T-FAP and 293T loaded tumor-bearing mice (n=4 per group). (B, D) Uptake of [68Ga]Ga-FAP-2286 and [18F]AlF-FAP-NUR at 60 min was assessed in 293T-FAP and 293T homozygous tumor-bearing mice (n=4 per group).Time-activity curves in A-D show the accumulation of radiotracer in the different organs from 5-120 min after injection. (E) Tumor background ratio (TBR) of the two tracers in different xenografts (including 293T-FAP and 293T hormonal mice) at 60 min. (F) TBR of both tracers in different xenografts (including A549-FAP and A549-loaded tumor-bearing mice) at 60 min. All data are expressed as mean % ID/g ± standard deviation (n=4).
3. Clinical Translation: First Human PET/CT Imaging
Clinical Equipment: Human imaging was performed using the UMI Panorama system, but pre-existing animal model data provided a key reference for clinical dose optimization.
Patient results: PET/CT of 2 cancer patients (breast cancer, lung cancer) showed primary tumor SUVmax of 21.20 and 14.19, muscle SUVmean of only 0.65-1.32, and TBR of 10.75-32.62, which verified the tumor-targeting ability of the probes in humans.

Figure 4 Clinical images of [18F]AlF-FAP-NUR PET/CT, HE staining results and FAP immunohistochemical staining of the patient's primary focus. (A) Patient with ductal carcinoma of the right breast. (B) Patient with invasive adenocarcinoma of the right lung. Scale bar, 50 μm.
Study Conclusions and Future Prospects
[18F]AlF-FAP-NUR combined with MadicLab high-resolution PET/CT demonstrated higher tumor-specific uptake and TBR values in animal models, and human imaging preliminarily confirmed its safety and efficacy. Although expanded clinical cohort validation is needed, this technology provides a new paradigm for early diagnosis and efficacy monitoring of FAP-positive tumors. In the future, it can be combined with the dynamic imaging capability of the MadicLab device to further optimize the pharmacokinetics of the probe and promote the development of precision nuclear medicine therapy.
Link to paper: DOI: 10.1186/s13550-024-01139-w
Equipment Support: MadicLab PSA071 PET/CT System (Shandong Madec Yinghua Technology Co., Ltd.)









