Selected Customer Papers | NWFU reveals the gut-brain axis mechanism of time-restricted eating to improve Alzheimer's disease
Release Date:2025-06-09 Number of views:329
Prof. Zhigang Liu's team from the School of Food Science and Engineering, North West Agriculture and Forestry University (NWAFU) published a study in the international journal iMeta, revealing for the first time the molecular mechanism by which time-restricted feeding (TRF) improves the cognitive function of Alzheimer's disease (AD) by regulating the intestinal flora - propionic acid - free fatty acid receptor 3 (FFAR3) axis and verifying the key processes of metabolites across the blood-brain barrier by using the MagicLab PET/CT technology, providing a new direction for non-pharmacological intervention in AD. With the help of MadicLab PET/CT, the key process of metabolites crossing the blood-brain barrier was verified, which provides a new direction for non-pharmacological intervention in AD.

MadicLab PET/CT's Central Role in Research
In exploring the neuroprotective mechanism of propionic acid (PA) against AD, the research team utilized the MadicLab Box 071 system to perform positron emission tomography (PET) imaging to validate the distribution and metabolic properties of PA in the brain:
• Validation of PA brain penetration: By intravenously injecting [18F]-labeled fluoropropionic acid ([18F]-FPA), PET images showed that PA could penetrate the blood-brain barrier and was significantly enriched in the hippocampus, cortex, striatum and other AD-related brain regions.
• Quantification of metabolic dynamics: PET data showed that PA metabolic signals in the brains of AD mice were significantly lower than those of healthy mice, and exogenous PA supplementation restored the metabolic activity of these regions, suggesting that there is a PA metabolic disorder in AD, and that supplementation of PA can reverse this abnormality.
• Validation of mechanism relevance: Combined with the FFAR3 knockdown assay, PET imaging confirmed that the neuroprotective effect of PA is dependent on FFAR3 receptor activation, providing direct imaging evidence for the “gut flora metabolite-brain axis” mechanism.
Research context and core scientific questions
The global prevalence of Alzheimer's disease (AD) continues to rise, and the association between intestinal flora disorders and AD pathology has been widely recognized, but how TRF, a non-invasive dietary intervention, exerts neuroprotective effects through the gut-brain axis is not clear. In this study, we aimed to analyze whether the ameliorative effect of TRF on AD is dependent on the intestinal flora and its metabolites, and to explore the key regulatory pathways.
Key Methods and Breakthrough Findings
1. Clinical Intervention: TRF Significantly Improves Cognitive Function in AD Patients
Design: Nine patients with AD underwent a 4-month time-restricted feeding intervention (8-hour daily feeding window), and cognitive changes were assessed using the Montreal Cognitive Assessment Scale (MoCA).
• Results: The overall cognitive scores of the patients were significantly improved (p<0.05), and the improvement in executive function was particularly significant; the level of fecal propionic acid (PA) was positively correlated with the cognitive scores (r=0.5677, p<0.0001), suggesting that PA may be a key metabolic marker for the efficacy of TRF.
2. Animal model: TRF alleviates AD pathology through intestinal flora.
5xFAD transgenic mouse model: 3-month TRF intervention significantly shortened the latency to escape from Morris water maze, reduced amyloid (Aβ) deposition in the brain by up to 30%, promoted microglia aggregation to Aβ plaques, and reduced the expression of inflammatory factors TNF-α and IL-1β.
• Flora clearance assay: After antibiotics cleared the intestinal flora, the cognitive improvement effect of TRF completely disappeared, confirming its dependence on the intestinal flora to mediate it. Supplementation with Bifidobacterium longum (B. pseudolongum) mimicked the effects of TRF, significantly reducing Aβ deposition and elevating PA levels. 3.
3. Multi-omics analysis: the central role of the B. pseudolongum-PA-FFAR3 pathway
Microbiome and metabolome analysis: TRF significantly enriched B. pseudolongum, and its abundance was positively correlated with PA level (r=0.68); PA entered the brain through the blood-brain barrier, activated the FFAR3 receptor, inhibited the phosphorylation of JNK, and up-regulated the neurotrophic factor BDNF.
• Molecular mechanism: In FFAR3 knockout mice, the neuroprotective effect of TRF completely disappeared, confirming that FFAR3 is a key target of TRF-PA pathway.
Figure : Propionic acid (PA) supplementation improves cognitive deficits in AD mice. (A) Schematic representation of groups treated with PA or carrier (n=7-8). (B) Escape latency. (C) Time spent in the target quadrant. (D) Immunohistochemical fluorescence images of Aβ deposition (green) and Iba-1+ (red) microglia in mouse cortex (n=3) (scale bar, 100 μm)
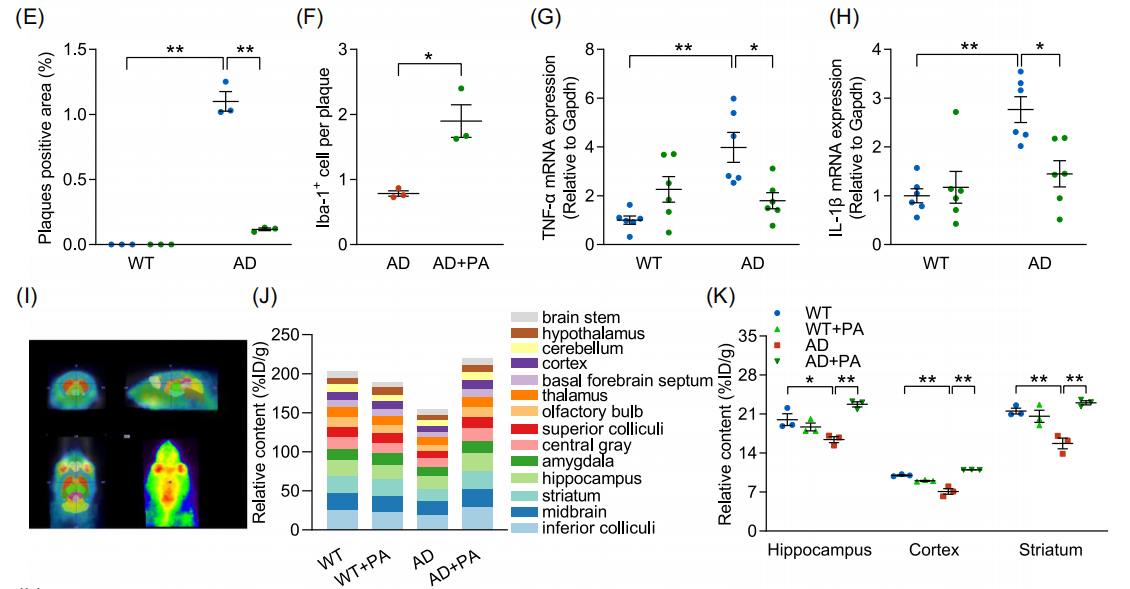
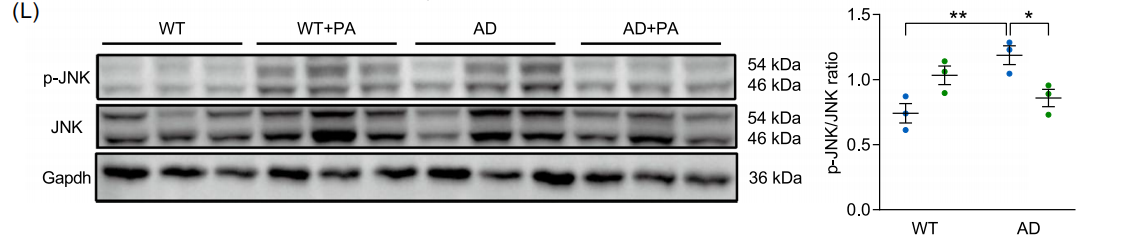
Fig. (E) Quantitative analysis of plaque-positive area. (F) Quantitative analysis of Aβ plaque-associated microglia. (G) mRNA levels of TNF-α (n=6). (H) mRNA levels of IL-1β (n=6). (I) Representative axial, sagittal and coronal positron emission tomography (PET) images showing PA brain uptake. (J) Brain uptake levels of [18F]-FPA (% ID/g) (n=3). (K) Quantitative PET analysis of [18F]-FPA in hippocampus, cortex and striatum. (L) Protein blot analysis of p-JNK and JNK (n=3). Data are expressed as mean ± standard error. *p<0.05, **p<0.01; two-way ANOVA and Tukey's multiple comparison test were used.
Translational potential and clinical significance
|
norm |
Key effects of the TRF intervention |
|---|---|
|
intestinal flora |
Enrichment of beneficial bacteria such as B. pseudolongum and remodeling of flora structure. |
|
metabolite |
Elevates fecal propionic acid (PA) levels, which positively correlates with cognitive scores and crosses the blood-brain barrier to modulate neuroinflammation. |
|
neuropathology |
Reduces Aβ deposition, inhibits microglia overactivation, and upregulates BDNF to improve neuronal survival. |
|
Advantages of non-pharmacological interventions |
Compared to traditional drugs, TRF is non-invasive, easy to comply with, and can achieve multi-targeted interventions by modulating the flora-metabolism axis. |
Potential applications
Probiotic therapy: B. pseudolongum may be a candidate for AD prevention or adjuvant therapy, and its PA-producing ability is a key characteristic.
Metabolite monitoring: Fecal PA level can be used as a non-invasive biomarker for early diagnosis of AD and evaluation of TRF efficacy, and the combination of brain imaging technology with MadicLab PET/CT can further enhance the accuracy of mechanism research.
-Combined intervention strategy: Combining TRF and FFAR3 agonists may enhance the neuroprotective effect and provide new ideas for personalized treatment of AD.
Challenges and future prospects
Current limitations: The clinical sample size is relatively small, and it is necessary to expand the cohort to verify the generalization of PA as a biomarker; the potential effect of long-term TRF on intestinal flora still needs to be observed.
Research directions: exploring the optimal intervention dose for B. pseudolongum, optimizing the brain-targeted delivery efficiency of PA analogues using MadicLab PET/CT, and validating the applicability of TRF in other neurodegenerative diseases.
Link to paper: DOI: 10.1002/imt2.70006
Equipment support: MadicLab Box 071 system (Shandong Madec Yinghua Technology Co., Ltd.)
Prof. Zhigang Liu's team from the School of Food Science and Engineering, North West Agriculture and Forestry University (NWAFU) published a study in the international journal iMeta, revealing for the first time the molecular mechanism by which time-restricted feeding (TRF) improves the cognitive function of Alzheimer's disease (AD) by regulating the intestinal flora - propionic acid - free fatty acid receptor 3 (FFAR3) axis and verifying the key processes of metabolites across the blood-brain barrier by using the MagicLab PET/CT technology, providing a new direction for non-pharmacological intervention in AD. With the help of MadicLab PET/CT, the key process of metabolites crossing the blood-brain barrier was verified, which provides a new direction for non-pharmacological intervention in AD.

MadicLab PET/CT's Central Role in Research
In exploring the neuroprotective mechanism of propionic acid (PA) against AD, the research team utilized the MadicLab Box 071 system to perform positron emission tomography (PET) imaging to validate the distribution and metabolic properties of PA in the brain:
• Validation of PA brain penetration: By intravenously injecting [18F]-labeled fluoropropionic acid ([18F]-FPA), PET images showed that PA could penetrate the blood-brain barrier and was significantly enriched in the hippocampus, cortex, striatum and other AD-related brain regions.
• Quantification of metabolic dynamics: PET data showed that PA metabolic signals in the brains of AD mice were significantly lower than those of healthy mice, and exogenous PA supplementation restored the metabolic activity of these regions, suggesting that there is a PA metabolic disorder in AD, and that supplementation of PA can reverse this abnormality.
• Validation of mechanism relevance: Combined with the FFAR3 knockdown assay, PET imaging confirmed that the neuroprotective effect of PA is dependent on FFAR3 receptor activation, providing direct imaging evidence for the “gut flora metabolite-brain axis” mechanism.
Research context and core scientific questions
The global prevalence of Alzheimer's disease (AD) continues to rise, and the association between intestinal flora disorders and AD pathology has been widely recognized, but how TRF, a non-invasive dietary intervention, exerts neuroprotective effects through the gut-brain axis is not clear. In this study, we aimed to analyze whether the ameliorative effect of TRF on AD is dependent on the intestinal flora and its metabolites, and to explore the key regulatory pathways.
Key Methods and Breakthrough Findings
1. Clinical Intervention: TRF Significantly Improves Cognitive Function in AD Patients
Design: Nine patients with AD underwent a 4-month time-restricted feeding intervention (8-hour daily feeding window), and cognitive changes were assessed using the Montreal Cognitive Assessment Scale (MoCA).
• Results: The overall cognitive scores of the patients were significantly improved (p<0.05), and the improvement in executive function was particularly significant; the level of fecal propionic acid (PA) was positively correlated with the cognitive scores (r=0.5677, p<0.0001), suggesting that PA may be a key metabolic marker for the efficacy of TRF.
2. Animal model: TRF alleviates AD pathology through intestinal flora.
5xFAD transgenic mouse model: 3-month TRF intervention significantly shortened the latency to escape from Morris water maze, reduced amyloid (Aβ) deposition in the brain by up to 30%, promoted microglia aggregation to Aβ plaques, and reduced the expression of inflammatory factors TNF-α and IL-1β.
• Flora clearance assay: After antibiotics cleared the intestinal flora, the cognitive improvement effect of TRF completely disappeared, confirming its dependence on the intestinal flora to mediate it. Supplementation with Bifidobacterium longum (B. pseudolongum) mimicked the effects of TRF, significantly reducing Aβ deposition and elevating PA levels. 3.
3. Multi-omics analysis: the central role of the B. pseudolongum-PA-FFAR3 pathway
Microbiome and metabolome analysis: TRF significantly enriched B. pseudolongum, and its abundance was positively correlated with PA level (r=0.68); PA entered the brain through the blood-brain barrier, activated the FFAR3 receptor, inhibited the phosphorylation of JNK, and up-regulated the neurotrophic factor BDNF.
• Molecular mechanism: In FFAR3 knockout mice, the neuroprotective effect of TRF completely disappeared, confirming that FFAR3 is a key target of TRF-PA pathway.
Figure : Propionic acid (PA) supplementation improves cognitive deficits in AD mice. (A) Schematic representation of groups treated with PA or carrier (n=7-8). (B) Escape latency. (C) Time spent in the target quadrant. (D) Immunohistochemical fluorescence images of Aβ deposition (green) and Iba-1+ (red) microglia in mouse cortex (n=3) (scale bar, 100 μm)


Fig. (E) Quantitative analysis of plaque-positive area. (F) Quantitative analysis of Aβ plaque-associated microglia. (G) mRNA levels of TNF-α (n=6). (H) mRNA levels of IL-1β (n=6). (I) Representative axial, sagittal and coronal positron emission tomography (PET) images showing PA brain uptake. (J) Brain uptake levels of [18F]-FPA (% ID/g) (n=3). (K) Quantitative PET analysis of [18F]-FPA in hippocampus, cortex and striatum. (L) Protein blot analysis of p-JNK and JNK (n=3). Data are expressed as mean ± standard error. *p<0.05, **p<0.01; two-way ANOVA and Tukey's multiple comparison test were used.
Translational potential and clinical significance
|
norm |
Key effects of the TRF intervention |
|---|---|
|
intestinal flora |
Enrichment of beneficial bacteria such as B. pseudolongum and remodeling of flora structure. |
|
metabolite |
Elevates fecal propionic acid (PA) levels, which positively correlates with cognitive scores and crosses the blood-brain barrier to modulate neuroinflammation. |
|
neuropathology |
Reduces Aβ deposition, inhibits microglia overactivation, and upregulates BDNF to improve neuronal survival. |
|
Advantages of non-pharmacological interventions |
Compared to traditional drugs, TRF is non-invasive, easy to comply with, and can achieve multi-targeted interventions by modulating the flora-metabolism axis. |
Potential applications
Probiotic therapy: B. pseudolongum may be a candidate for AD prevention or adjuvant therapy, and its PA-producing ability is a key characteristic.
Metabolite monitoring: Fecal PA level can be used as a non-invasive biomarker for early diagnosis of AD and evaluation of TRF efficacy, and the combination of brain imaging technology with MadicLab PET/CT can further enhance the accuracy of mechanism research.
-Combined intervention strategy: Combining TRF and FFAR3 agonists may enhance the neuroprotective effect and provide new ideas for personalized treatment of AD.
Challenges and future prospects
Current limitations: The clinical sample size is relatively small, and it is necessary to expand the cohort to verify the generalization of PA as a biomarker; the potential effect of long-term TRF on intestinal flora still needs to be observed.
Research directions: exploring the optimal intervention dose for B. pseudolongum, optimizing the brain-targeted delivery efficiency of PA analogues using MadicLab PET/CT, and validating the applicability of TRF in other neurodegenerative diseases.
Link to paper: DOI: 10.1002/imt2.70006
Equipment support: MadicLab Box 071 system (Shandong Madec Yinghua Technology Co., Ltd.)









